Regulation governing certain matters relating to official control of the production, treatment and placing on the market of foodstuffs of animal origin (Animal Food-Monitoring Regulation-Tier-LMÜV)
Unofficial table of contents
Animal LMÜV
Date of completion: 08.08.2007
Full quote:
" Animal food surveillance ordinance of 8 August 2007 (BGBl. I p. 1816, 1864), as last amended by Article 2 of the Regulation of 11 November 2010 (BGBl. 1537).
| Status: |
Last amended by Art. 2 V v. 11.11.2010 I 1537 |
For more details, please refer to the menu under
Notes
Footnote
(+ + + Text evidence from: 15.8.2007 + + +)
Heading: letter abbreviation inserted. by Art. 3 No. 1 V v. 11.5.2010 I 612 mWv 21.5.2010
The V was established as Article 3 of the V v. 8.8.2007 I 1816 by the Federal Ministries of Food, Agriculture and Consumer Protection and for Health in agreement with the Federal Ministries of Economy and Technology, for Environment, Nature Conservation and Reactor safety, finances and the judiciary with the approval of the Bundesrat. She's gem. Art. 23 of this V entered into force on 15 August 2007.
Unofficial table of contents
§ 1 Scope
This Regulation shall govern the official supervision of the production, treatment and placing on the market of foodstuffs of animal origin, and the implementation and implementation of European Community legislation or acts European Union in the field of the monitoring of transport of foodstuffs of animal origin.
Unofficial table of contents
§ 2 Definitions
(1) For the purposes of this Regulation:
-
1.
-
Foodstuffs of animal origin: products of animal origin as defined in Annex I, point 8.1, indent 1 of Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for Food of animal origin (OJ L 327 EU No 55, No L 139, p. 22), as amended,
-
2.
-
prohibited substances or products: substances or products the use of which is prohibited in the case of live animals within the meaning of Article 4 (1) (1) of the Food and Feed Code,
-
3.
-
application of prohibited substances or products or use of authorised substances or products for areas of application for which the application is excluded, in the case of live animals, within the meaning of Article 4 (1) (1) of the the Food and Feed Code,
-
4.
-
Residues: residues of substances with a pharmacological effect and their conversion products, as well as of other substances which may be transferred to food of animal origin and which may affect human health,
-
5.
-
Consignment: a group of live animals of the same species and age group which have been reared simultaneously in the same holding under the same conditions of holding and feeding.
(2) In addition, the definitions of
-
1.
-
Article 2 of Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs (OJ L 327, 30.4.2004, p. EU No L 139 p. 1, No L 226 p. 3) and
-
2.
-
Annex I to Regulation (EC) No 853/2004
accordingly.
Unofficial table of contents
§ 3 Official auxiliaries
(1) The competent authority may only appoint persons to official technical assistants who:
-
1.
-
the successful completion of a primary school or at least equivalent educational attainment,
-
2.
-
physical and health fitness through a medical certificate,
-
3.
-
the required reliability by means of an official certificate and
-
4.
-
the qualification by an official certificate, in accordance with paragraph 2, of the successful training and examination referred to in
-
a)
-
Annex I, Section III, Chapter IV, point (B), (5) or (8) of Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 on specific procedural rules for the official control of human consumption , certain products of animal origin (OJ C EU No L 139 p. 206, No OJ L 226 p. 83),
-
b)
-
§ 3 para. 2 sentence 3 of the meat inspector regulation of 30 June 1992 (BGBl. 1227), in the version valid until 14 August 2007, or
-
c)
-
§ 4 (2) sentence 3 of the Regulation on Poultrymeat Inspectors of 24 July 1973 (BGBl. 899), as amended up to 14 August 2007
(2) The evidence of the qualification referred to in paragraph 1 (4) shall be issued in the case of persons who have been more than
-
1.
-
have not taken part in training activities in accordance with Annex I, Section III, Chapter IV (B) (6) of Regulation (EC) No 854/2004, or
-
2.
-
two years have not been acting as an official technical assistant.
Proof of competence may be re-provided by passing an official inspection to determine whether the knowledge required from a theoretical and practical point of view, as referred to in Annex I, Section III, Chapter IV (B), is required. Article 5 of Regulation (EC) No 854/2004 is still in place. (3) The national governments are empowered to comply with the provisions of Regulation (EC) No
-
1.
-
the implementation of the training and examination referred to in Annex I, Section III, Chapter IV (B) of Regulation (EC) No 854/2004, and the issuing of an official certificate, and
-
2.
-
the implementation of training measures referred to in Annex I, Section III, Chapter IV (B), (6) and (7) of Regulation (EC) No 854/2004, and
-
3.
-
the carrying out of the verification referred to in the second sentence of paragraph 2
to the Commission.
Unofficial table of contents
§ 4 Schlachthofpersonnel
(1) The competent authority may, upon request, authorise the staff of a slaughterhouse
-
1.
-
in the official supervision of the production of meat of poultry or of hats, under the conditions laid down in Article 5 (6) (a), in conjunction with Annex I, Section III, Chapter III (A) of Regulation (EC) No 854/2004, the activities described there shall be replaced by official technical assistants, or
-
2.
-
in accordance with Article 5 (6) (b) in conjunction with Annex I, Section III, Chapter III (B) of Regulation (EC) No 854/2004, to carry out certain tests or the sampling of samples for certain laboratory tests.
(2) In the case referred to in paragraph 1 (1), the official veterinarian shall have, pending the establishment of detailed rules for the performance tests referred to in Annex I, Section III, Chapter III (A), sub-letter (a) (3) of Regulation (EC) No 854/2004, of each consignment Slaughtered poultry and bodily caves of at least 300 animals distributed throughout the entire consignment must verify that the slaughterhouse staff has carried out the delegated tasks properly. (3) Prior to the initial deployment of the Slaughterhouse staff referred to in paragraph 1 (2) shall have the competent authority within the framework of a theoretical and a practical examination of the success of the training provided for in Annex I, Section III, Chapter III, point B, of Regulation (EC) No 854/2004.
Unofficial table of contents
§ 5 Meat-hygiene measures in the context of zoonoses and disease eradication programmes
The competent authority may require slaughter under programmes for the eradication or control of animal diseases or of zoonotic agents as defined in Annex I, Section II, Chapter III, point 7 of Regulation (EC) No 854/2004, approve that
-
1.
-
the slaughter is to be carried out following the other slaughterings;
-
2.
-
slaughtering must be carried out in a spatially separate way from the other slaughterings if there is a suspicion that the animal being examined is affected by a contagious disease which can be transferred to the slaughterhouse.
In the case of the first sentence of paragraph 1, special precautions shall be taken to protect the slaughter staff. (2) The official veterinarian shall, if necessary, take further measures, taking into account the specific characteristics of the pathogens, in order to: Avoid contamination of other animals or the meat of other animals. In the justified individual case, the Federal Institute for Risk Assessment may be involved. (3) After completion of the slaughterings referred to in paragraph 1, the official veterinarian shall have appropriate cleaning and disinfection of all premises, equipment and equipment. to arrange equipment which may have been contaminated with pathogens of animal diseases or zoonoses in the course of the slaughtering referred to in paragraph 1.
Unofficial table of contents
§ 6 Meat examination and examination on trichinae before submission of small quantities of wild game
(1) In the case of small quantities of wild animals, which are declared pursuant to Article 4 (2), first sentence, point 1 or 2, also in conjunction with paragraph 3, of the animal food hygiene regulations for official meat inspection or for official examination on trichinae has been
-
1.
-
the official post-mortem inspection referred to in Annex I, Section IV, Chapter VIII (A), in conjunction with Section II, Chapter V, point 1, of Regulation (EC) No 854/2004, as amended, or
-
2.
-
the official investigation into trichinae referred to in Article 2 (3), third subparagraph, in conjunction with Annex I, Chapter I or Annex II, and Annex III to Commission Regulation (EC) No 2075/2005 of 5 December 2005, with specific rules for the official Post-mortem inspection of trichinae (OJ C EU No OJ L 338, p. 60), as amended
, For the purposes of the assessment of the results of the studies referred to in the first sentence, Annex I, Section IV, Chapter VIII (B) and Chapter IX (C) of Regulation (EC) No 854/2004 shall apply. (2) The competent authority may be a hunter who shall: the holder of a valid annual hunting licence; and
-
1.
-
In accordance with § 2b of the Animal Food Hygiene Regulations Wild, for the purpose of use as a food for domestic consumption, or
-
2.
-
in accordance with Article 3 (1), first sentence, point 5 of the animal food hygiene regulations, the supply of small quantities of wild game or meat of wild game,
in the case of wild boar or badger, the collection of samples for examination on trichinae referred to in the first sentence of paragraph 1, point 2. A transfer in accordance with the first sentence may be effected only if:
-
1.
-
the hunter has been trained by the competent authority for the performance of that activity; and
-
2.
-
there are no facts justifying the assumption that the hunter does not possess the reliability required for this activity.
Unofficial table of contents
§ 7 ante-mortem inspection in the delivery of small quantities of meat from poultry or lagomorphs
The competent authority shall have at least two agricultural holdings in which small quantities of fresh meat of poultry or hawfish are delivered in accordance with Article 3 (1), first sentence, of the animal food hygiene regulation to carry out an annual ante-mortem inspection in the form of regular health monitoring of the stock. Sentence 1 shall not apply in the cases of § 3 (1) sentence 2 of the Animal Foodstuffs Hygiene Regulations.
Unofficial table of contents
§ 7a Official investigations in the extraction of meat for domestic consumption
(1) In the case of animals which have been declared for official examination in accordance with Article 2a (1) of the Animal Food Hygiene Regulations,
-
1.
-
the official ante-mortem inspection referred to in Annex I, Section I, Chapter II, Part B, and Section II, Chapter III, also in conjunction with Section IV, Chapter IV, Part A, or Chapter VII, Part A, and in Chapter IX, Parts A, E and F of Regulation (EC) No 854/2004,
-
2.
-
the official post-mortem inspection referred to in Annex I, Section I, Chapter II, Part D, and Section II, Chapter V, point 1, also in conjunction with Section IV, Chapters I, II, III, IV, Part B, or Chapter VII, Part B, and with Chapter IX, Part A, B and D to F of Regulation (EC) No 854/2004,
-
3.
-
the official investigation into trichinae in accordance with Annex I, Section IV, Chapter IX, Part C of Regulation (EC) No 854/2004, as amended, in conjunction with the third subparagraph of Article 2 (3), in conjunction with Annex I, Chapter I or II, and Annex III to Regulation (EC) No 2075/2005
, By way of derogation from point 3 of the first subparagraph, the competent authority may carry out the examination on trichinae referred to in the third subparagraph of Article 2 (3) in conjunction with Annex I, Chapter III, of Regulation (EC) No 2075/2005. (2) In the case of large wild game, which is the case in accordance with § 2b the animal food hygiene regulations have been declared for official post-mortem inspection or for the official examination on trichinae, § 6 (1) shall apply mutagenically.
Unofficial table of contents
§ 7b Official investigations in wild farms with low production volume of shell game
(1) In the context of the authorisation of the slaughter or killing of wild game for the production of meat intended for human consumption at the place of provenance in accordance with Annex III, Section III, point 3 of Regulation (EC) No 853/2004, the competent authority may: shall also authorise the slaughter or killing in wild farms with a low production volume, by way of derogation from Article 5 (1) (b), in conjunction with Annex I, Section I, Chapter II, Part B, point 1 (b) of Regulation (EC) No 854/2004 may also be carried out if the official ante-mortem inspection, notwithstanding Article 5 Point 1 (b) in conjunction with Annex I, Section I, Chapter II, Part B, point (1) (b) of Regulation (EC) No 854/2004, has not been carried out within 24 hours, but within 28 days of slaughter, provided that: Person with the knowledge of an informed person in accordance with Annex III, Section IV, Chapter I of Regulation (EC) No 853/2004 immediately before slaughter or killing, has found that the animal to be slaughtered or killed does not Behavioural disturbances are observed and a suspicion of harmful effects by the environment (2) In the case referred to in paragraph 1, the official or authorised veterinarian who carried out the ante-mortem inspection shall, in point 5 of the health certificate referred to in Annex I, Section IV, Chapter X, Part B, of the Regulation (EC) No 854/2004 to delete the second indent of the declaration. The authorization referred to in Annex III (III) (3) to Regulation (EC) No 853/2004 may be granted under the conditions set out in paragraph 1, even if the holding does not exceed the procedures laid down in Annex III, Section III, point 3 (e), of the Regulation (EC) No 853/2004. (3) Wild farms with a low production volume within the meaning of this provision are wild farms which do not slaughter more than 50 pieces of wild game every year or kill meat for human consumption. or for slaughter.
Unofficial table of contents
§ 8 Labelling of the fitness for health
(1) In the case of meat of domestic ungulates which have been slaughtered outside a slaughterhouse, the health marking referred to in Annex I, Section I, Chapter III, point 7, of Regulation (EC) No 854/2004 shall have a (2) Small quantities of large wild animals in which no post-mortem inspection is carried out in accordance with the first sentence of Article 6 (1) but which, in accordance with Article 6 (1), first sentence, No. 2 on Trichiners , and not in accordance with Article 6 (1), second sentence, in conjunction with Annex I, Section IV, Chapter VIII (B), of the Regulation (EC) No 854/2004 has been declared unfit for human consumption, shall be marked on the free-lying meat parts or the breast-coat with a mark according to the form and content of the model of Appendix 1 (2). Sentence 1 shall not apply in the case of Section 6 (2), first sentence, point 2. (3) Small quantities of large wild animals, which are examined in accordance with Article 6 (1), first sentence, No. 1 and not in accordance with Article 6 (1) sentence 2 in conjunction with Annex I, Section IV, Chapter VIII (B) of the Regulation (EC) No 854/2004 has been declared unfit for human consumption by means of a mark according to the form and content of the model of Appendix 1 (3), in accordance with Annex I, Section I, Chapter III (2) (b) of Regulation (EC) No 854/2004. (4) meat within the meaning of Article 4 (1) of Commission Regulation (EC) No 2076/2005 of 5 June 2005. December 2005 laying down transitional arrangements for the implementation of Regulations (EC) No 853/2004, (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council and amending Regulations (EC) No 853/2004 and (EC) No 853/2004 N ° 854/2004 (OJ L 344, EU No 83) must be marked with a mark according to the form and content of the model of Appendix 1 (4). (5) The meat of wild game,
-
1.
-
in the case of an official authorisation pursuant to Section 7b (1), the ante-mortem inspection shall not be carried out within 24 hours before slaughter or the slaughter at the place of provenance shall be subject to the conditions laid down in Article 7b (2) sentence 2 has been approved and
-
2.
-
that has not been declared unfit for human consumption,
by way of derogation from Annex I, Section I, Chapter III, point 3 of Regulation (EC) No 854/2004, shall be marked with a mark in the form and content of the design of Appendix 1, point 5. (6) Meat, except for meat of poultry or haedening, which shall: in accordance with Annex I, Section II, Chapter V, point 1 or section IV, Chapter VIII, point B, of Regulation (EC) No 854/2004, has been declared unfit for human consumption by means of a mark according to the form and content of the model of Appendix 1, point 5, as referred to in paragraph 1 (7) Labelling materials which are to be labelled prior to 15 August 2007 , and which do not meet the requirements of the relevant content of the samples of Appendix 1, may be continued until 31 December 2010.
Unofficial table of contents
§ 9 Resumption of the raw milk supply
(1) The arrangement of the suspension of the milk supply referred to in the second sentence of Chapter II of Chapter II of Annex IV to Regulation (EC) No 854/2004 shall be repealed if the results of two representative samples taken at intervals of at least four days are Herdenmilk has been shown to show that the raw milk corresponds to the limit values set out in Appendix 2. The arrangement of the suspension of the milk supply may also be lifted if:
-
1.
-
in the third month following the initial notification of the competent authority, raw milk has complied with the limit values set out in Annex 2;
-
2.
-
the food business operator can prove, by means of appropriate documentation, that it has taken measures to comply with the levels of somatic cells and germs, and
-
3.
-
it has been demonstrated by the result of a representative sample of herd milk that the raw milk corresponds to the limit values set out in Appendix 2.
The samples referred to in sentences 1 and 2, point 3, shall be taken out and examined at the request of the food business operator by the competent authority or a body designated by the competent authority. (2) The competent authority shall immediately suspend the remorel. to order the supply of raw milk from the producer ' s holding, if:
-
1.
-
in the month in which the order has been lifted in accordance with paragraph 1, it is established that the raw milk does not comply with the limit values set out in Appendix 2, or
-
2.
-
the following month, it is established that the raw milk does not meet the criteria set out in Annex III, Section IX, Chapter I, Part III, point 3 (a), of Regulation (EC) No 853/2004.
Unofficial table of contents
§ 10 residue monitoring
The competent authority shall, within the framework of the implementation of Annex I, Section I, Chapter II, Point (F) (1) (c) of Regulation (EC) No 854/2004
-
1.
-
in the case of at least 2% of all commercially slaughtered calves and at least 0.5 per cent of all other commercially slaughtered ungulates, to be taken from official samples and to be examined for residues; and
-
2.
-
Official samples of live animals within the meaning of § 4 (1) (1) of the Food and Feed Code and of foodstuffs of animal origin according to the provisions of the residue monitoring plan drawn up pursuant to § 2 No. 10 of the BVL Act To investigate residues.
Official samples referred to in the first sentence shall be marked for the protection of identity by means of information on the species, type and method of sampling, quantity of the sample, sex of the animal and the origin of the animal or food. (2) The competent authority shall: (3) In the case of live animals within the meaning of Article 4 (1) (1) of the Food and Feed Code from a holding or from those animals, Food has been repeatedly found to have fixed maximum levels for authorised Substances listed in Annex I to Council Directive 96 /23/EC of 29 April 1996 on control measures in respect of certain substances and residues thereof in live animals and animal products and repealing Directives 85 /358/EEC, and 86 /469/EEC and Decision 89 /187/EEC and 91 /664/EEC (OJ L 206, 22.7.1989, p. EC No 10) or the conversion products thereof, the competent authority has, over a period of at least six months, increased the number of official samples of live animals within the meaning of Article 4 (1) (1) of the Food and Agriculture Committee ( (4) If from the competent authority for live animals within the meaning of Article 4 (1) (1) of the Food and Feed Code of Food and Feed Producer holding or a livestock trading or transport company an order pursuant to section 41 (3) of the the Food and Feed Code has been adopted by the competent authority over a period of at least 12 months to a greater extent by official samples of live animals within the meaning of Article 4 (1) (1) of the Food and Feed Code (1) of the Food and Feed Code ( (5) Where the result of the examination of an official sample referred to in paragraph 1 or the first sentence of Article 41 (3) or (5) of the food and food products is found to be Animal feed code as a result of the result of the investigation of a law pursuant to § 43 (1) Article 2 of the Food and Feed Code, the competent authority has to initiate an examination of the official sample by the national reference laboratory. (6) When facts exist, the competent authority shall: , the official veterinarian shall, in the context of the implementation of the Directive, reliably indicate that slaughter animals have been subjected to irregular treatment or that they have been given prohibited substances or products, or that there is a sufficient suspicion of them. of Annex I, Section II, Chapter III, point 6, of Regulation (EC) No 854/2004
-
1.
-
the slaughter of these animals shall be carried out separately from the other slaughterings; and
-
2.
-
Provisional seizure of carcasses and by-products of slaughter and the official samples required for the clarification of the suspicion of laboratory tests in accordance with Annex I, Section I, Chapter II, point (F) (1) (c) of Regulation (EC) No 854/2004.
(7) Where there are facts which reliably indicate that animals approved for slaughter have been supplied with a pharmacological effect and that the animals are to be slaughtered before the expiry of the prescribed withdrawal period, or the official veterinarian has ordered the postponement of the slaughter. The period of time for the slaughter to be postponed shall be such that the prescribed waiting period is complied with and the maximum quantities fixed shall not be exceeded. (8) By way of derogation from paragraph 7, the official veterinarian may be slaughtered where reasons of animal welfare or operational circumstances so require. In this case, meat and by-products of slaughter shall be seized and official samples taken for laboratory tests in accordance with Annex I, Section I, Chapter II, point F (1) (c) of Regulation (EC) No 854/2004. (9) The seizure is to the extent that the laboratory tests have shown that the maximum quantities fixed are not exceeded.
Unofficial table of contents
Section 11 Transitional provisions
By way of derogation from Section 6 (2), the provisions of Section 22a (1) sentence 2 and 3 of the Meat Hygiene Act as amended by the Notice of 30 June 2003 (BGBl) shall be until 20 November 2010. 1242, 1585), in the version valid until 6 September 2005.
Unofficial table of contents
Appendix 1 (to § 8)
Stamp for the identification of the fitness for the health
(Fundstelle: BGBl. I 2007, 1868-1969;
with regard to of the individual amendments. Footnote)
-
1.
-
Stamp for the consumption of meat from domestic animals, which have been slaughtered outside a slaughterhouse
-
2.
-
Stamp for the consumption of meat from large wild game, which has been subjected to the investigation on trichinae
-
3.
-
Stamp for the consumption of meat from large wild game, which has been subjected to the post-mortem inspection
-
4.
-
Stamp for the consumption of meat from slaughterhouses within the meaning of Article 4 of Regulation (EC) No 2076/2005
-
5.
-
Stamp for the seabed meat of wild game according to § 7b
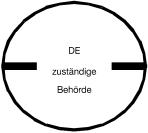
___ 3.5 cm ___ |
-
6.
-
Stamp for meat unfit for human consumption
Unofficial table of contents
Appendix 2 (to § 9)
Limit values for the repeal of the order referred to in Annex IV, Chapter II, point 2, first sentence, of Regulation (EC) No 854/2004
(Fundstelle: BGBl. I 2007, 1870)
Germ count at + 30 ° C (per ml) Somatic cells (per ml)
| Raw cow's milk |
≤ 100,000 |
≤ 400,000 |
Raw milk of other
Animal species |
≤ 1 500 000 |
|
| Raw milk of other animal species intended for the production of raw milk products without heat treatment |
≤ 500,000 |
|